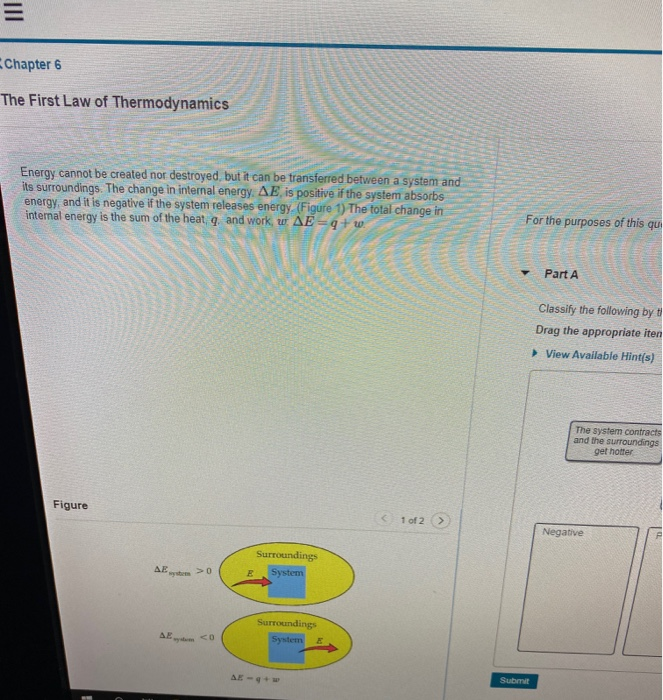
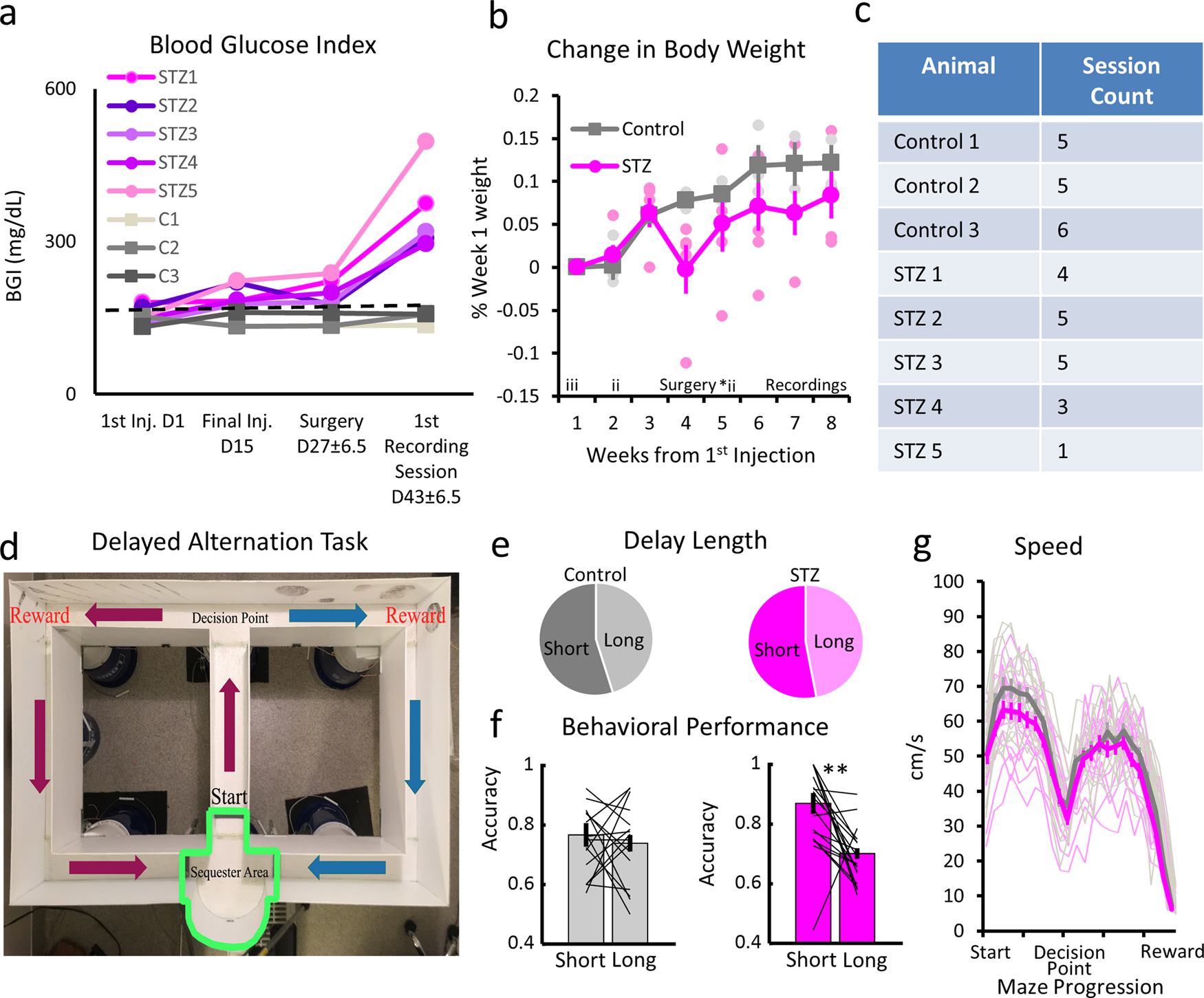
Classify The Following By The Sign Of δe For The System
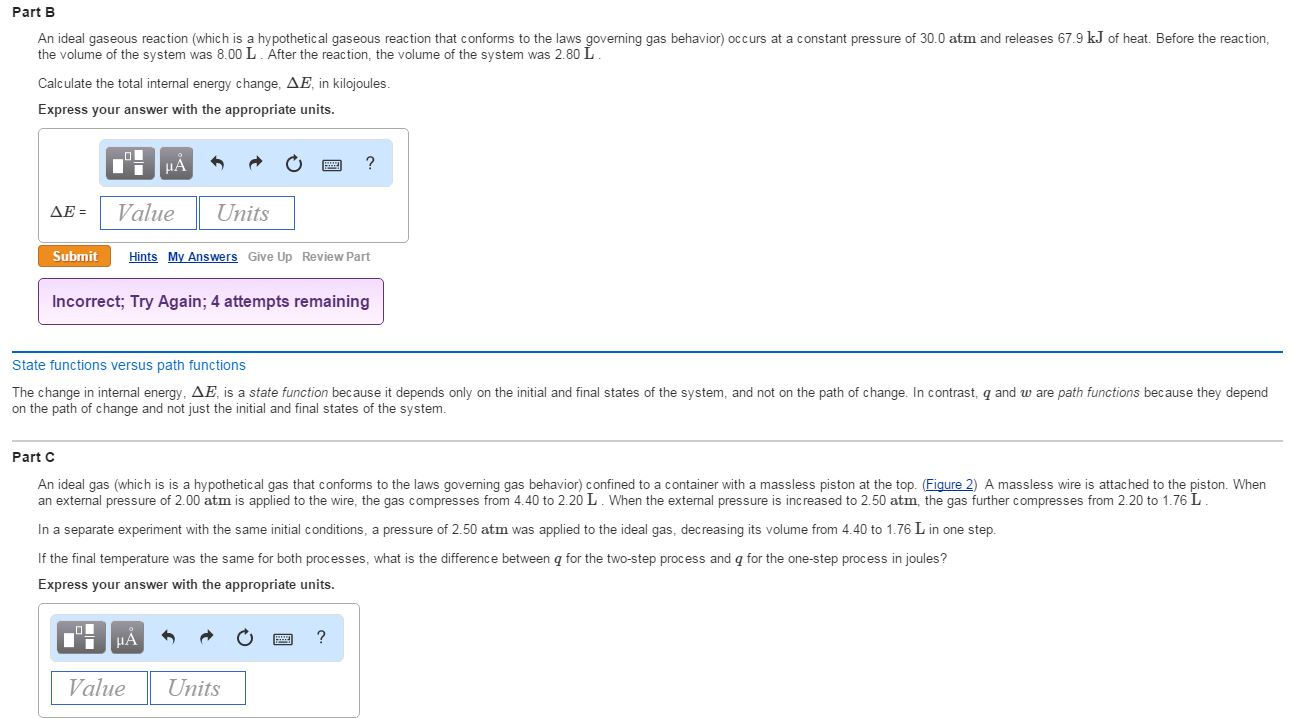
Classify the following by the sign of δe for the system. Classify the following by the sign of ΔE for the system. Chemistry questions and answers. An ideal gaseous reaction which is a hypothetical gaseous reaction that conforms to the laws governing gas behavior occurs at a constant pressure of 500 atm and releases 649 kJ of heat.
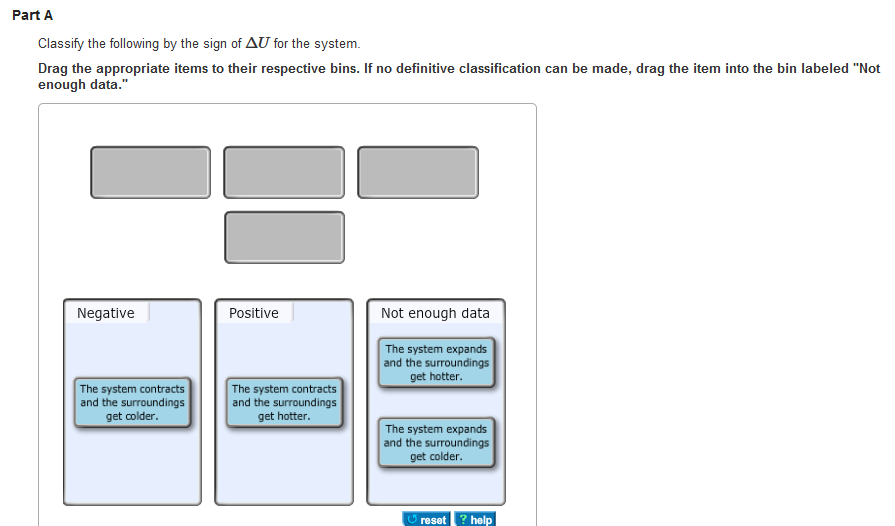
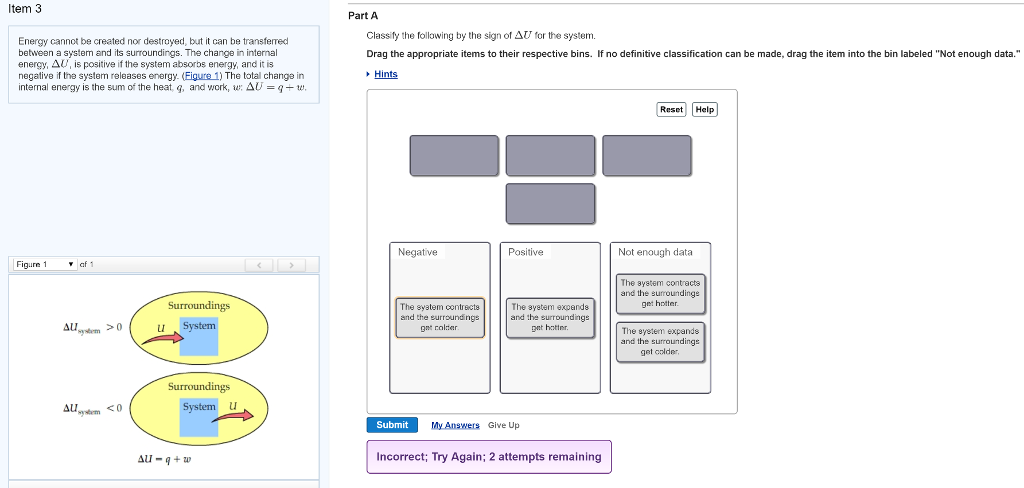
The lengths of the arrows rep-resent the relative magnitudes of q and w. Classify the following by the sign of deltaU for the system. C the system absorbs 725 kJ of heat from the surroundings while its volume remains constant assume that only P-V work can be done.
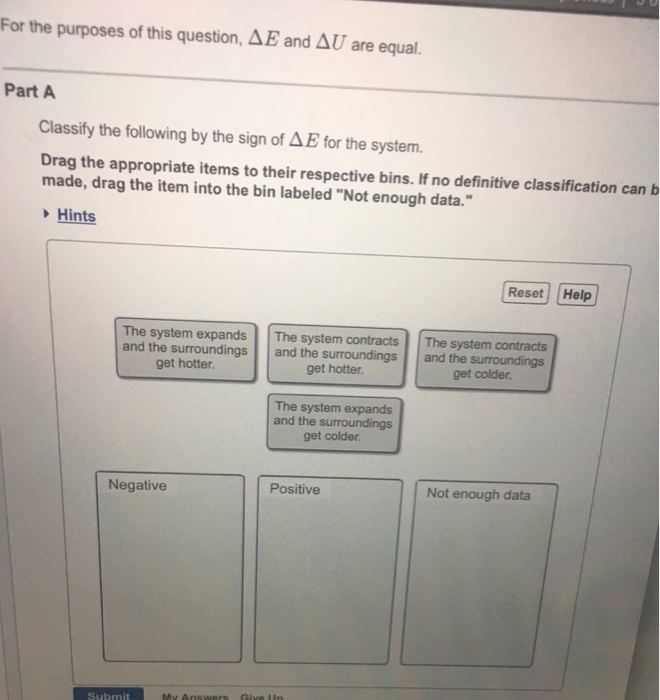
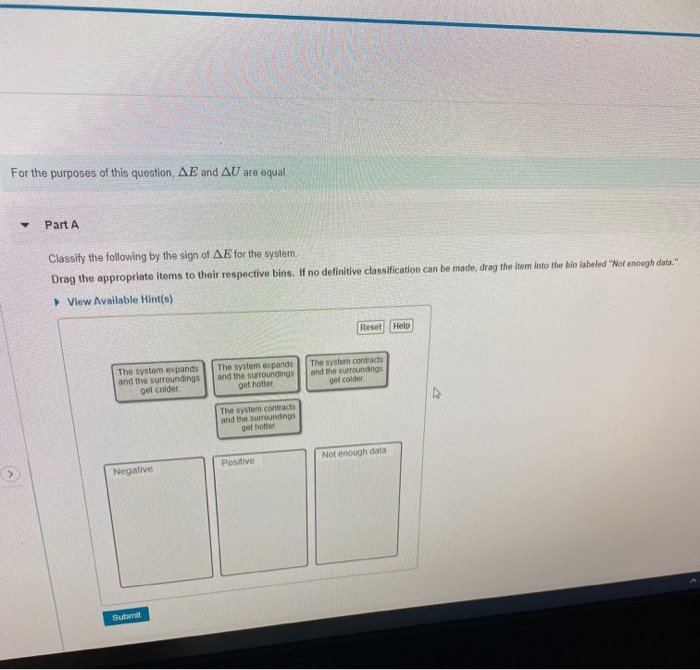
The system expends and the surrounding gets colder. 962 Indicate the direction of heat transfer between the system and the surroundings classify the following processes as endo- or exothermic and give the sign of ΔH. A Which of these processes is endothermic.
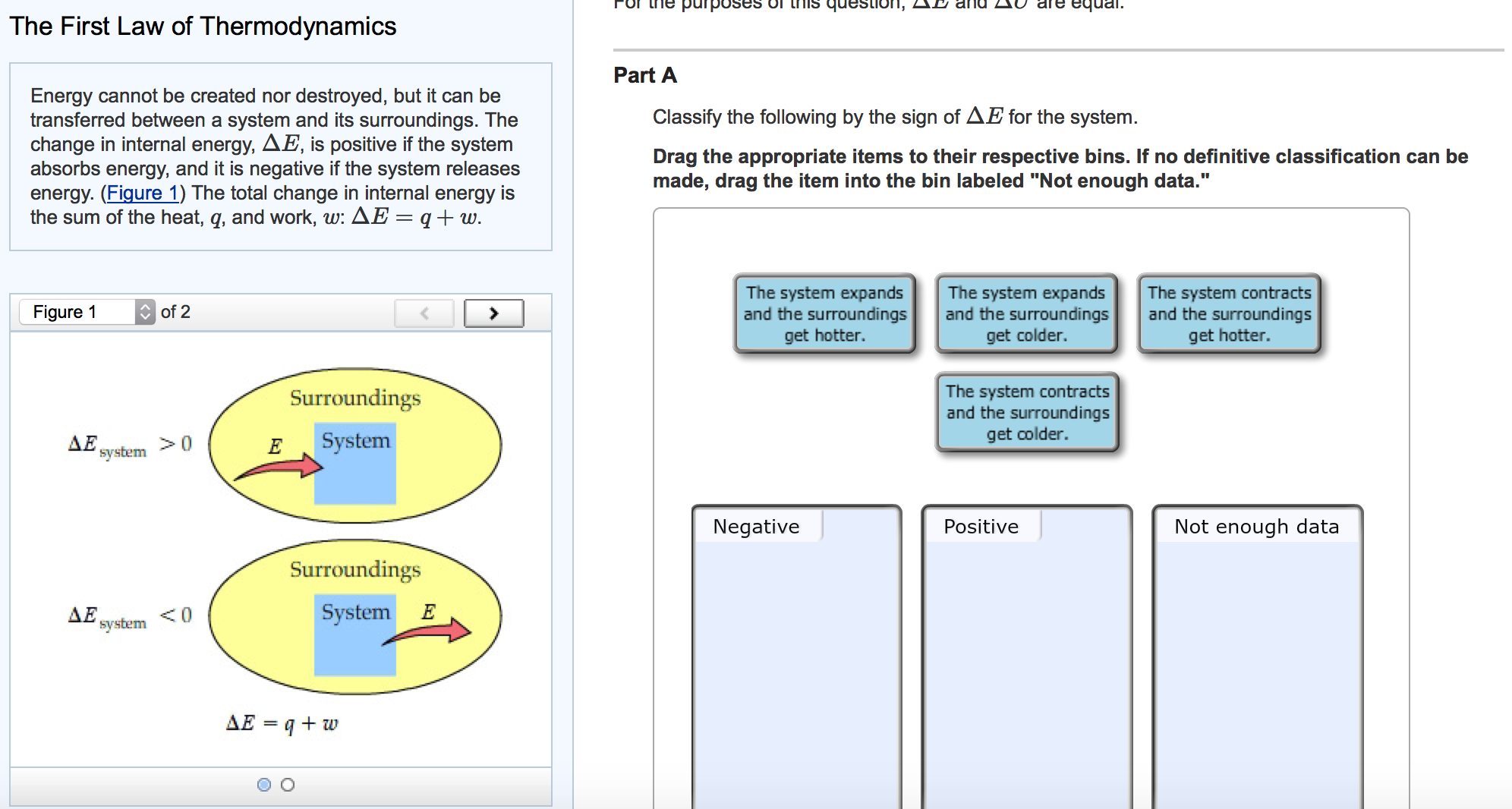
Predicting the Sign of Δ. The following can be classified into these three categories negativepositive not enough data 1. The system expands and the surroundings get hotter.
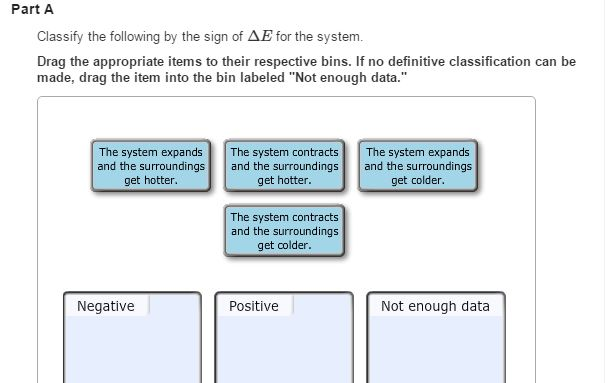
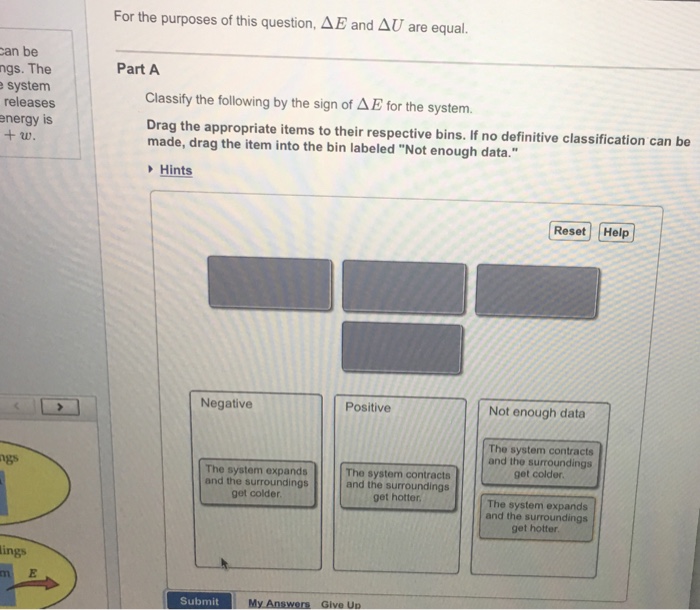
Classify the following by the sign of ΔE for the system. The system contracts and the surroundings get hotter. How much work has been done in joules if the cylinder goes from a volume of 0180 liters to.
If no definitive classification can be made drag the item into the bin labeled Not enough data please neeh help asap. If no definitive classification can be made drag the item into the bin labeled Not enough data The system expands The system contracts The system expands and the surroundings and the surroundingsand the. The system expands and the surroundings get hotter.
Classify the following by the sign of deltaU for the system. Indicate whether each of the following processes produces an increase or decrease in the entropy of the system.
Chemistry questions and answers.
Classify the following by the sign of ΔE for the system. The system contracts and the surroundings get hotter. C the system absorbs 725 kJ of heat from the surroundings while its volume remains constant assume that only P-V work can be done. ΔE is Positive or negative I. A Evaporating rubbing alcohol from your skin. The system expands and the surroundings get cooler. The system expands and the surroundings get hotter. Part A Classify the following by the sign of AE for the system. B a system releases 661 kJ of heat to its surroundings while the surroundings do 440 kJ of work on the system.
The system expands and the surroundings get colder. Drag the appropriate items to their respective bins. The lengths of the arrows rep-resent the relative magnitudes of q and w. The system contracts and the surroundings get colder. Classify the following by the sign of deltaU for the system. If no definitive classification can be made drag the item into the bin labeled Not enough data Energy cannot be created nor destroyed but it can be transferred between a system and its surroundings. 40 minutes Number of Problems.


























Post a Comment for "Classify The Following By The Sign Of δe For The System"